Clinpal
Clinpal is an end to end platform for clinical trials and is provided as a series of stand alone modules.
History
I was the first hired person in the company. At that time there were only 3 co-founders, one of them who was an engineer. So from the very beginning, I was working closely with developer on building clinical trial management and electronic data
capture platform.
Challenge
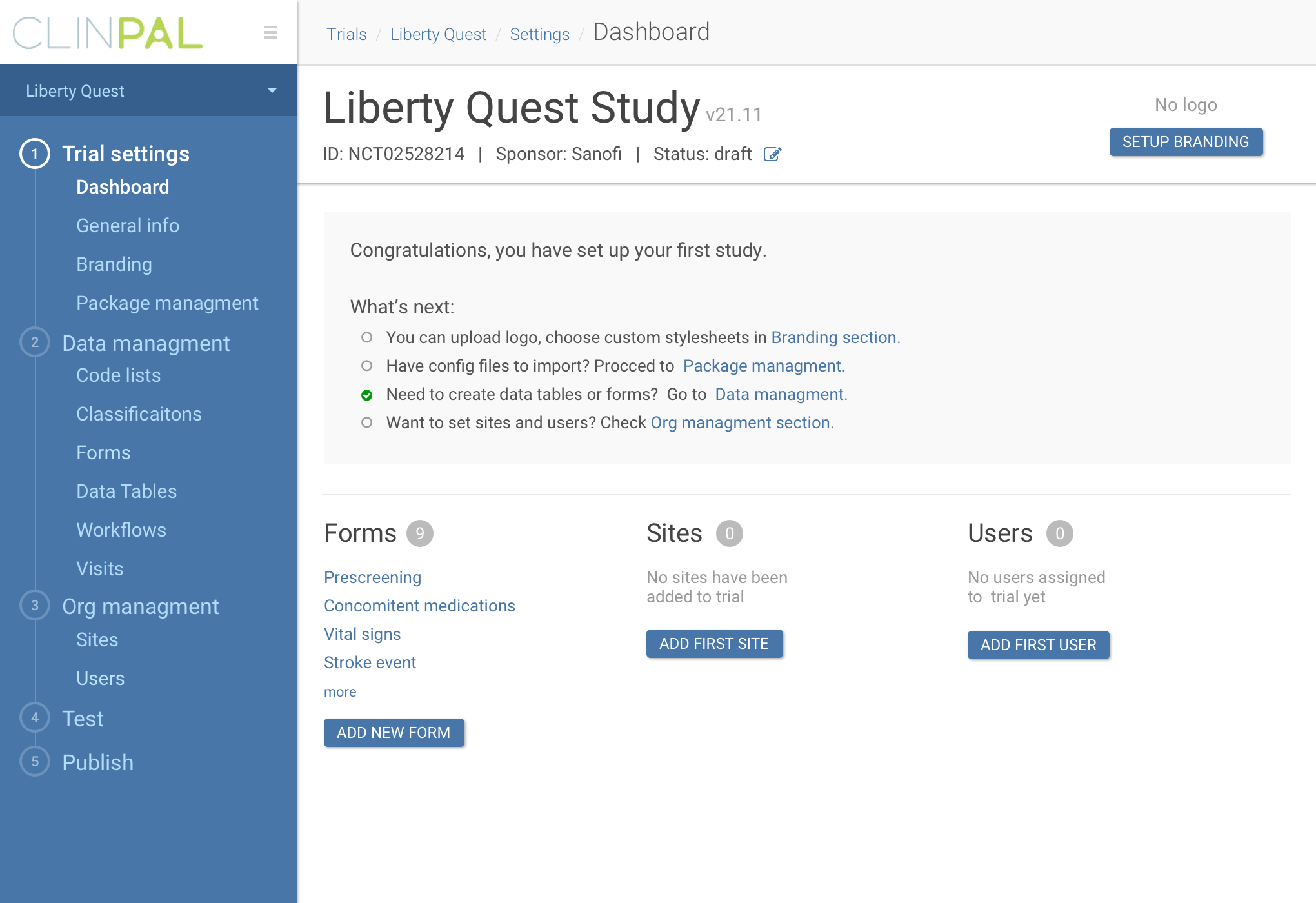
The idea of the platform was to connect patients to upcoming trials all over the world using search by condition, provide patient engagement through the trial lifecycle, give pharma companies powerful tool to build study documents and workflows, and
allow study monitors and investigators work with data outcomes easily.
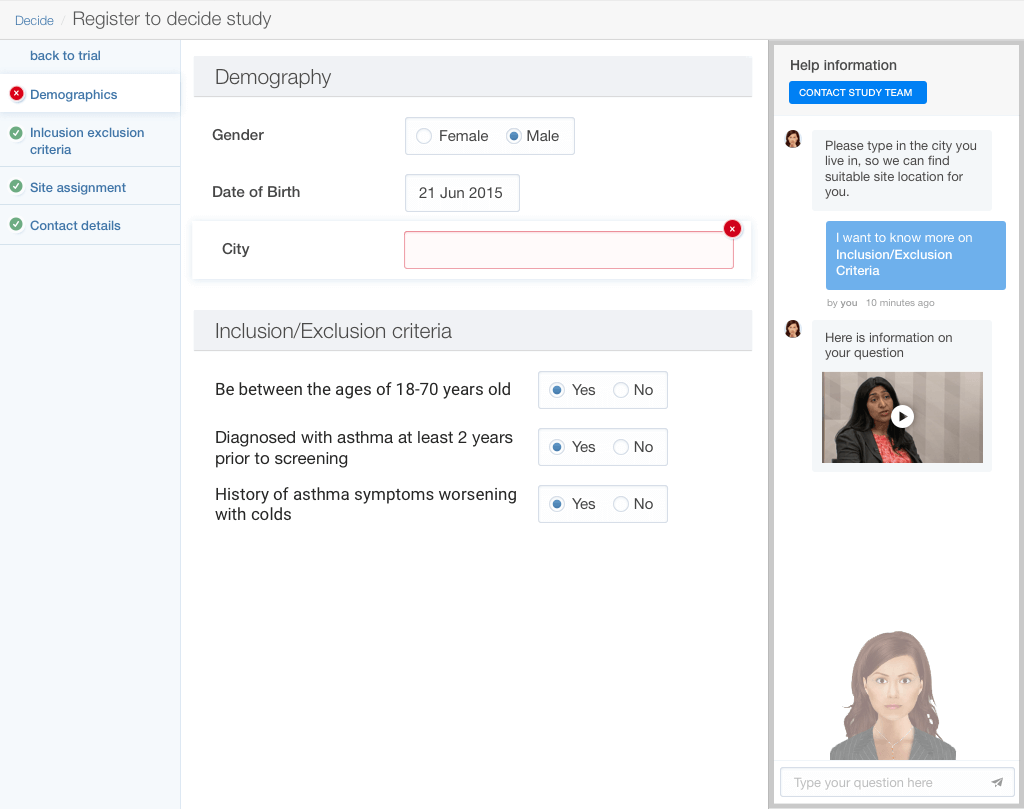
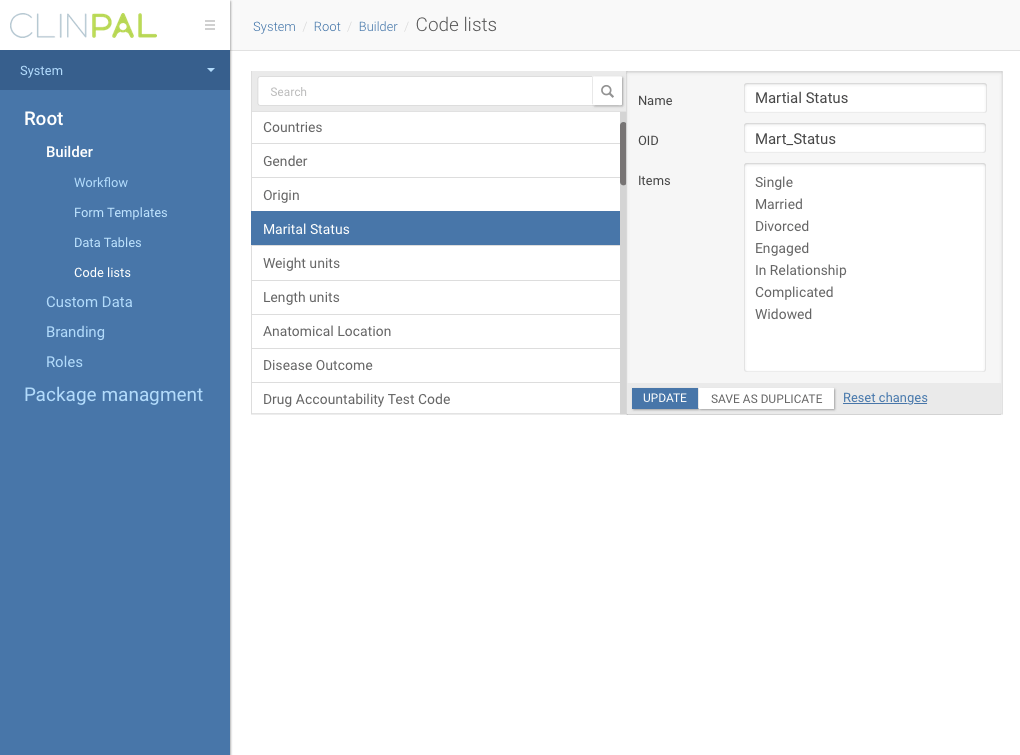
Trial builder dashboard
Process
The whole project was constant challenge for me. At first, I had to learn a lot of things from the clinical trial world. The clinical industry is very regulated, so it put a lot of constraints on architecture and UI solutions we implemented. For
example, pharma companies can’t see patient personal information, so all this information is blinded to clinical study admins, they can’t reach patients directly to discuss something.
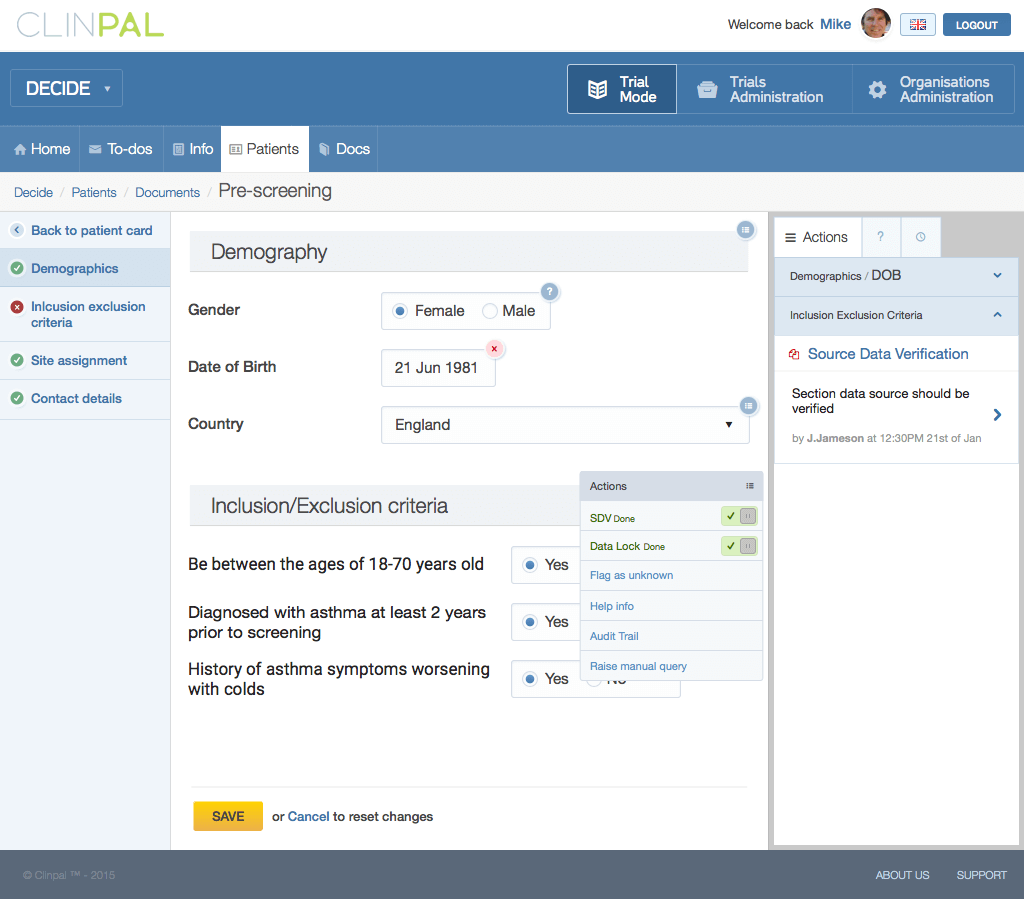
Investigator working with patient data
At the same time due to the nature of clinical study, all data should be captured in proper format, so we can’t really allow freeform comments coming from patients to study investigators. For example, if patient is complaining about pain, he can’t
just message his doctor in a trial and tell him about it. There are special kind of documents and follow up events to cover such cases. So all such things reflected on solutions that I made in UI.
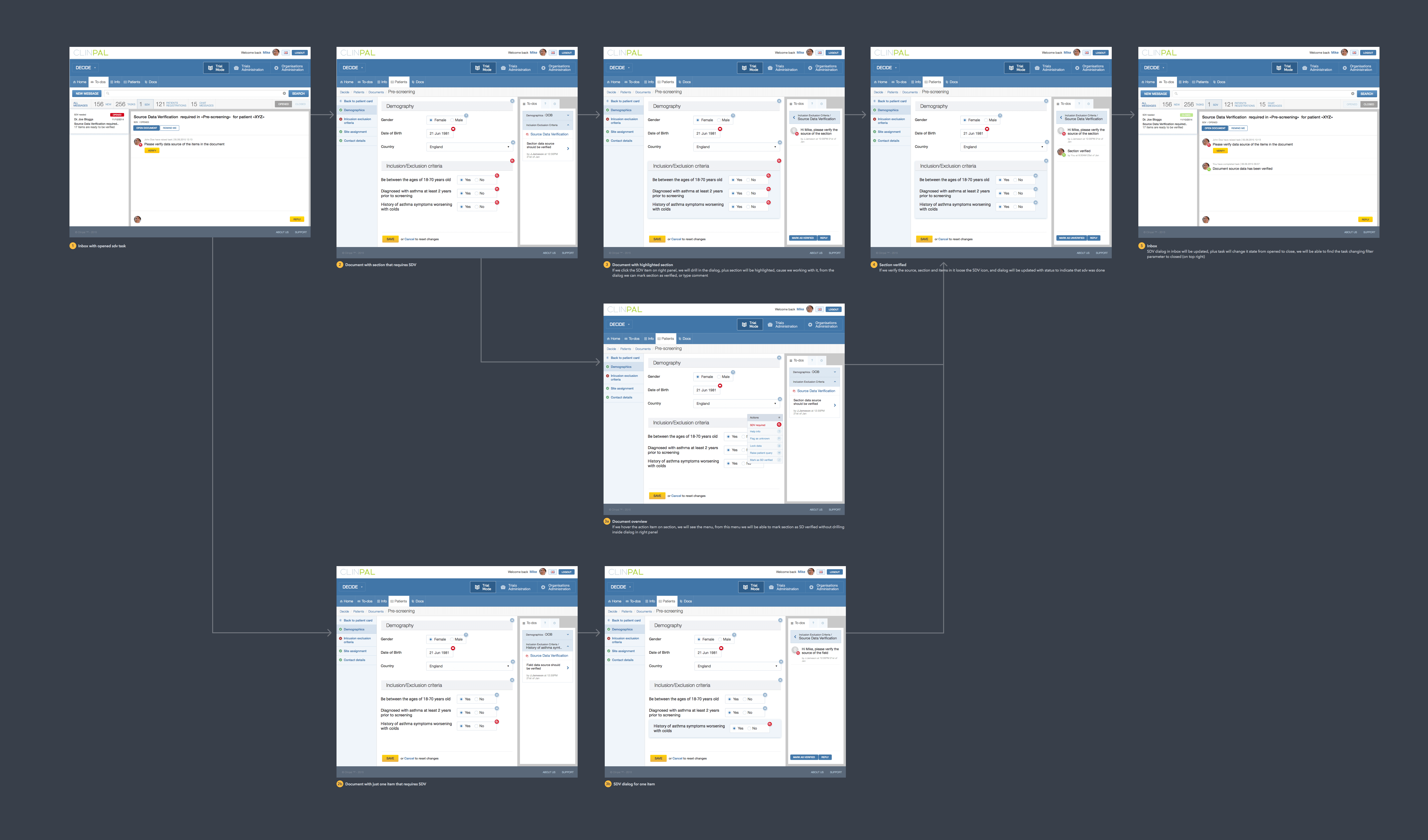
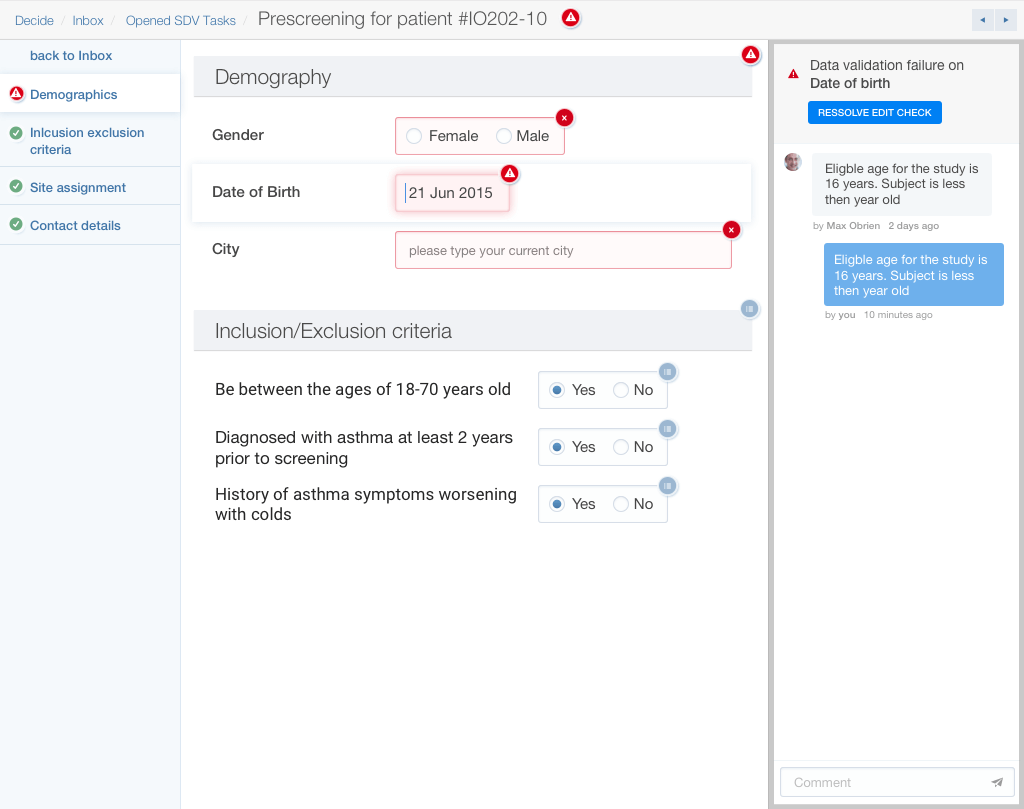
Source data verification flow
Next problem appeared the nature of the product itself, it was pivoted a couple of times, and mostly driven by first customers who were trying to get product with different functionality. So we had to build very complex but flexible configurable
platform.
At the very beginning it was easy to move new UI into production because feedback loop was short - client- founders - me&CTO.
Then we started to receive user's feedback, do our own UX research using lookback.io and got proper development processes using Kanban. Unfortunately due to the specific of clinical industry, we were not allowed to contact most of our users
directly, so feedback we've got was limited.
Another constraint affecting UI decision was the choice of the tech stack - which was GWT at that time. Which appeared hard in terms of dealing with complex UI interactions. So I had to do some shortcuts in UI, trying to match my mocks with basic GWT
components.
Unfortunately my knowledge of front-end development at that time was far from perfect, and I made some mistakes on building HTML/LESS UI kit for the app, which ended up too complex from my current point of view:)
And because software is done and we have limited resources, it's hard to get front-end debts into roadmap, but i am trying to push it to our product manager so we can fix that in some near future hopefully:)
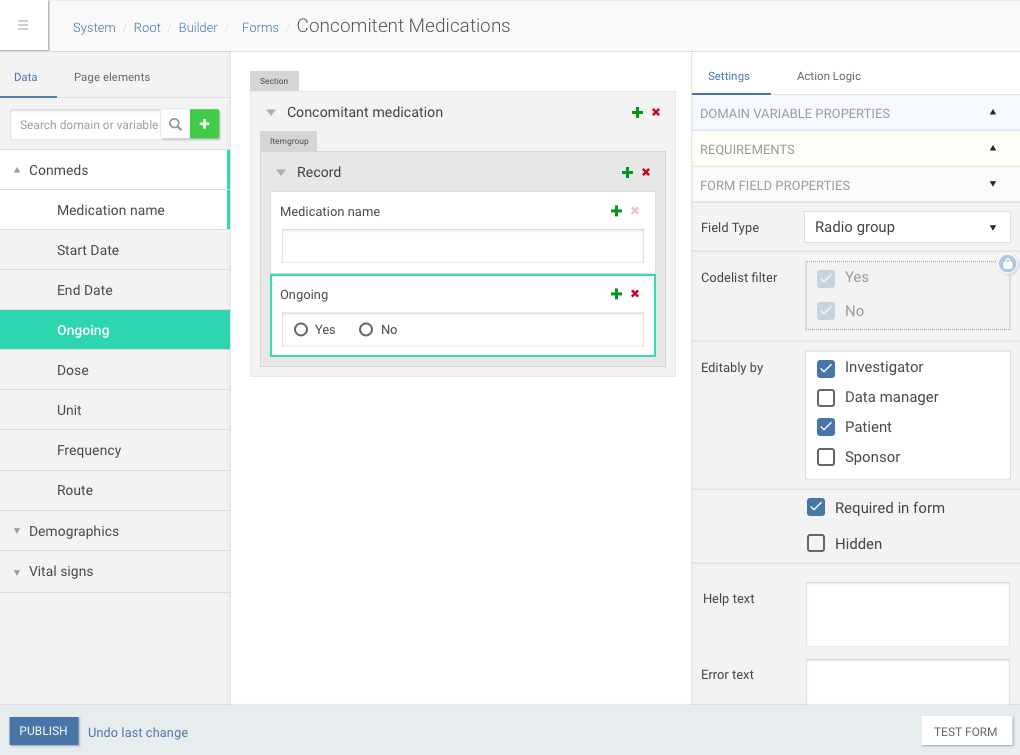
Our platform allowed pharma companies to build complex forms and trial timelines for patients using configurable XML files, which was fine on early stage, but no scalable enough, cause we had to do all trial configuration in-house.
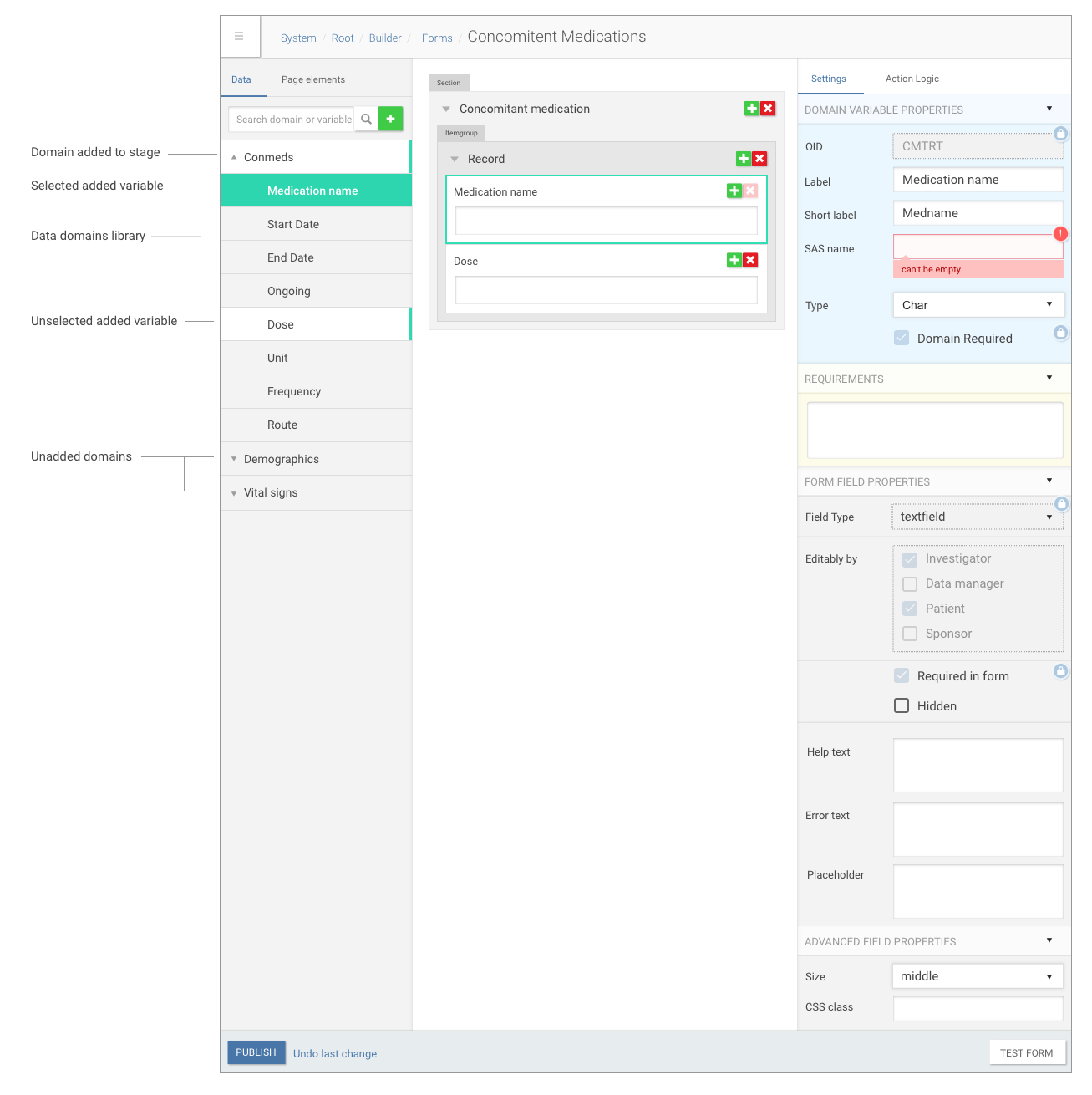
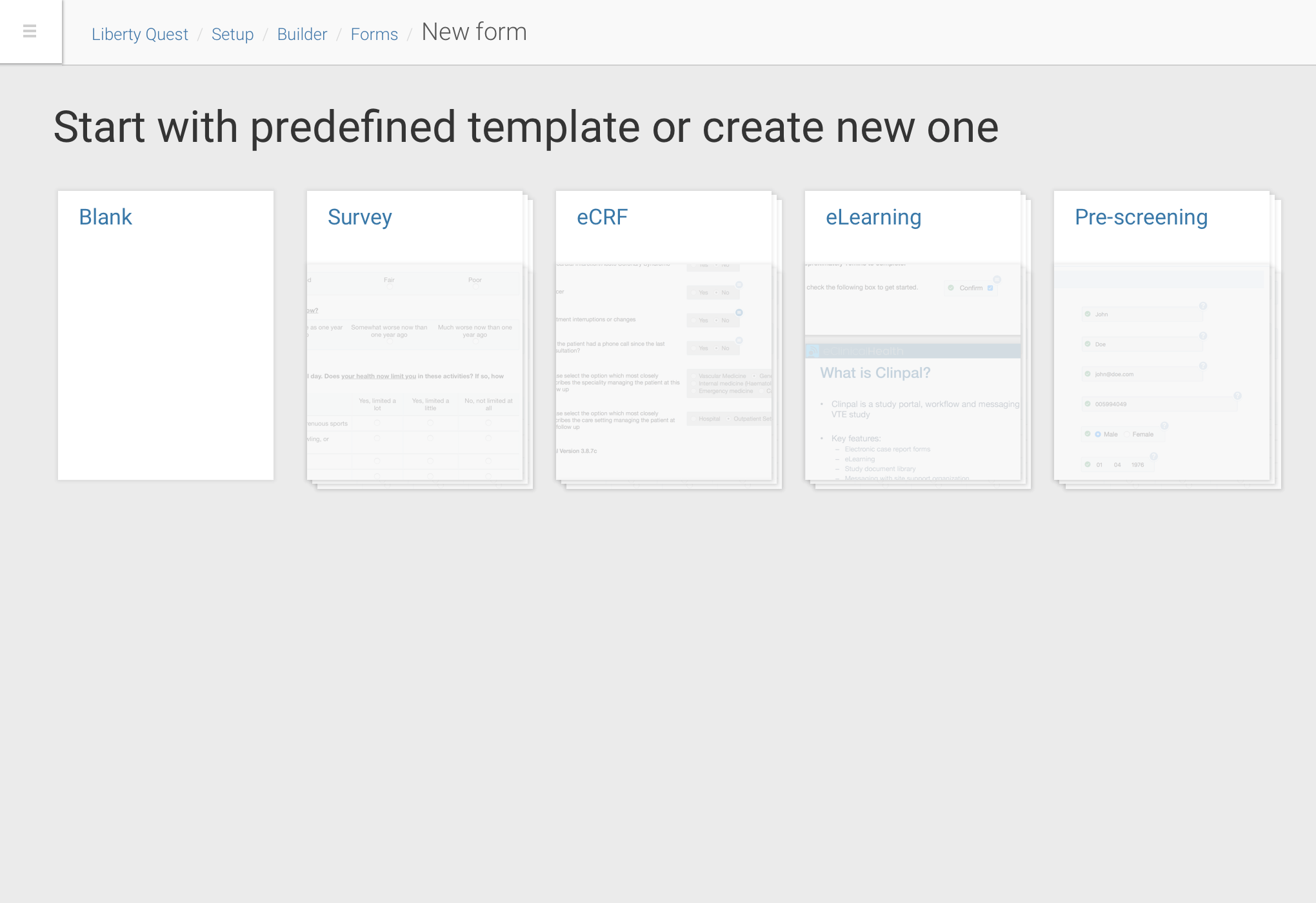
Trial document builder interface
So a year ago, at 2016 we started building new trial management tool based on Angular tech to help people without technical knowledge to build studies easily.
My front-end skills were powerful enough at this time to make UI kit properly and easy customisable for our needs. I learned Angular on basic level, which allowed me to produce reusable HTML/CSS components that I was getting straight to project, so
developers can build the platform on top of them.
Angular is a very flexible platform from front-end side of things, especially in comparison with GWT. So it allowed me to make rich UI with complex drag and drop interactions, dynamic content creation which I first prototype in Framer.
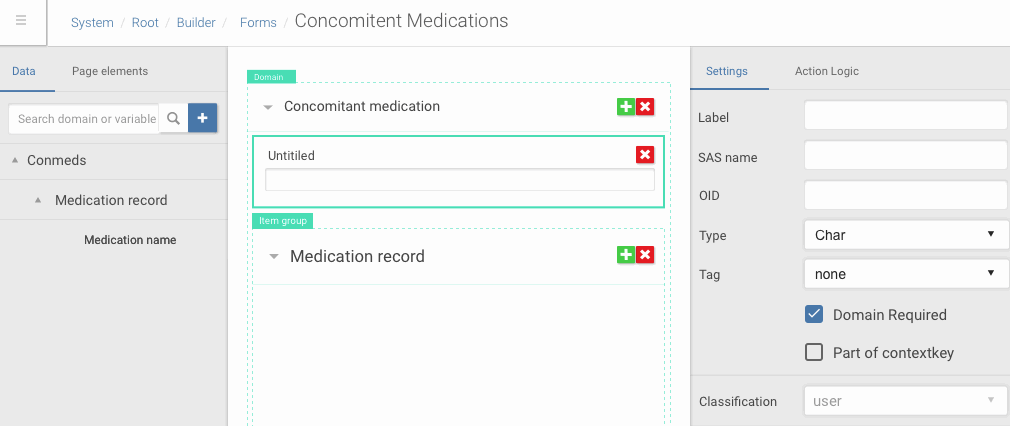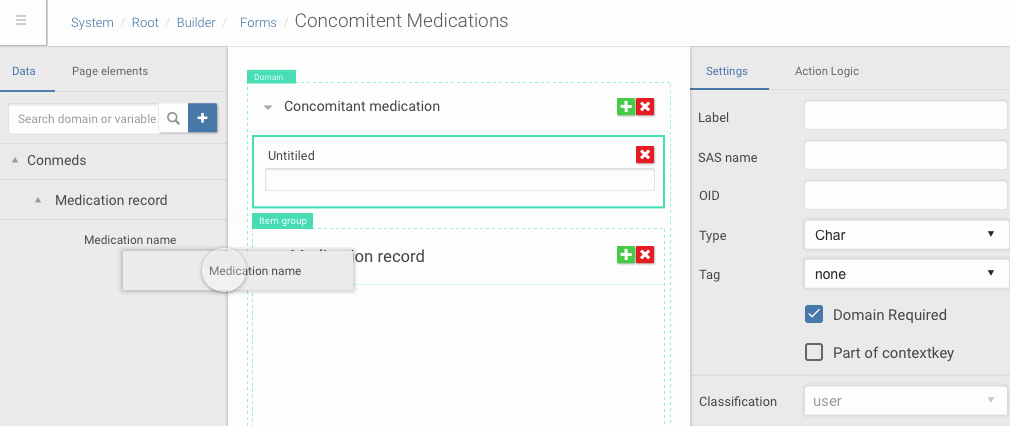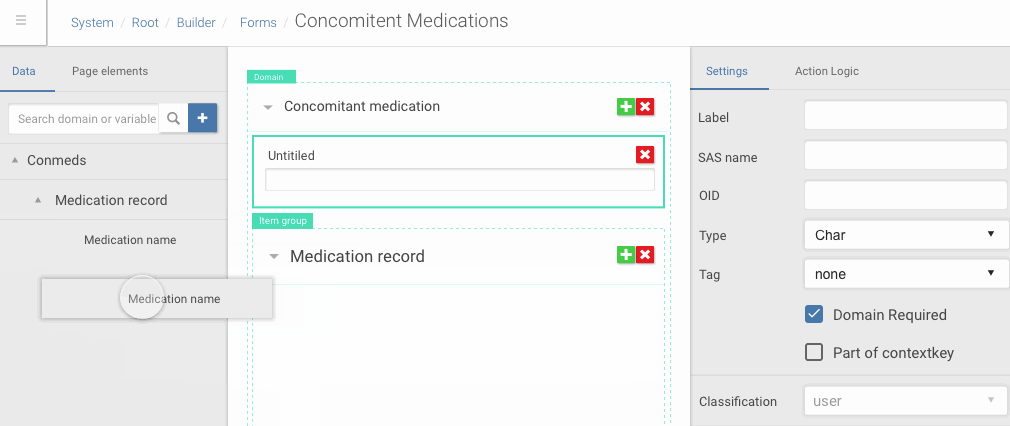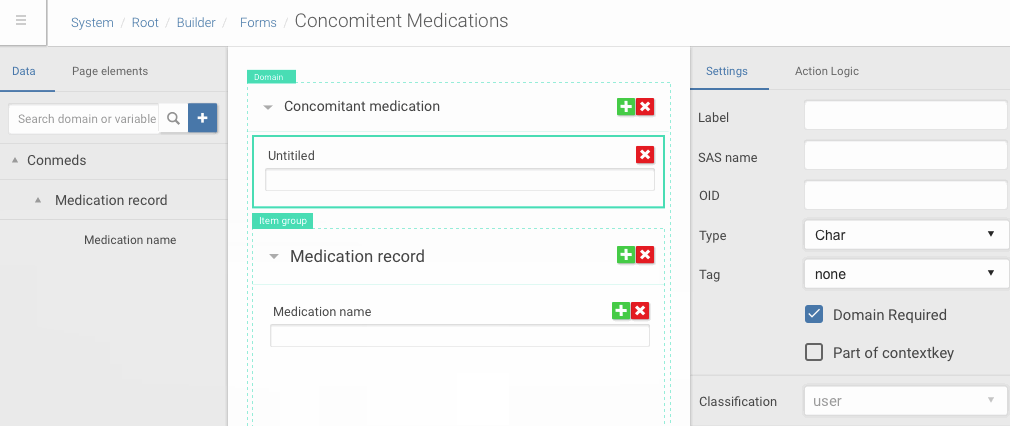
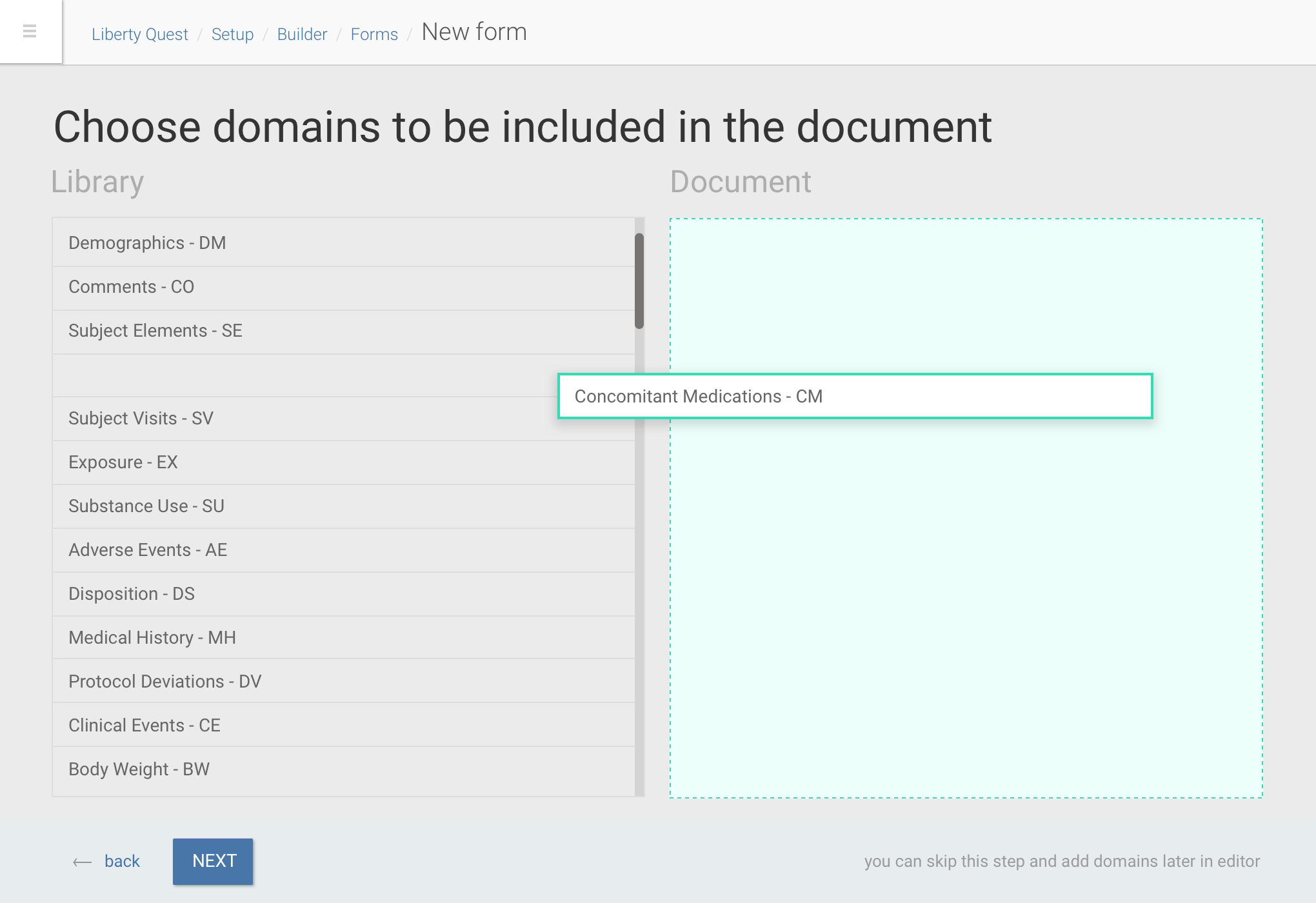
Drag and drop of data dictionary items into forms
Framer allowed me quickly iterate complex one screen interactions based on feedback from the team and clients.
Outcome
I think it was the most challenging project in my life, I got a huge amount of technical skills. It allowed me to progress from the guy who was scared by Terminal and “SVN” 🙃 to guy who was building stuff locally, deploy on the test server, mess
with Mongo DB and get hands dirty in java classes sometimes. Also, project helped me to develop my designer's skills - cause working with lots of constraints is really challenging thing and pushing you to limits sometimes😎
In terms of the product itself, it pretty successful right now on the market. It's still growing and has some challenges, but our company survived first two years when 99% percents of startups die. And during this process, we helped hundreds of
patients with their health condition connecting them to modern clinical trials. We launched
first remote clinical study in the world.